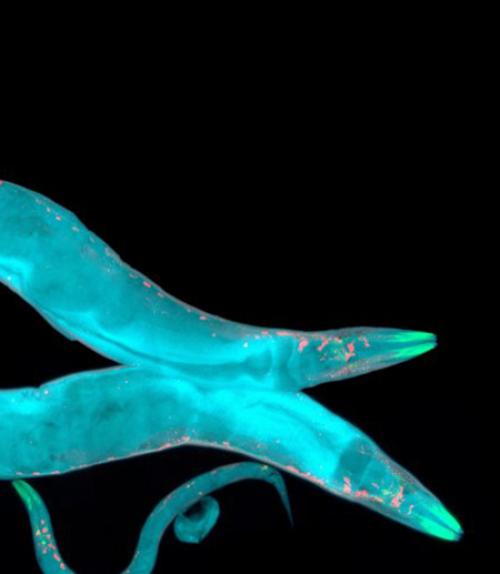
What can a humble worm, only one millimeter long, tell us about human physiology? Plenty, says Jun (Kelly) Liu, Molecular Biology and Genetics. Liu has spent years studying cell fate decisions and cell signaling pathways in Caenorhabditis elegans, a transparent nematode that was the first multicellular organism to have its whole genome sequenced.
"An adult C. elegans hermaphrodite has a fixed number of somatic (nonreproductive) cells—exactly 959,” Liu says. “It’s one of the best organisms to study molecular mechanisms controlling development at single cell resolution, because it’s transparent, and we know the entire lineage history of those 959 cells, starting from the single, fertilized egg.”
How Do Cells Produce Various Organs in the Body?
While scientists knew the worm’s cell lineage, their understanding of the mechanism behind the differentiation of all its cells was rudimentary. Liu aimed to change that. In her early work, she focused on a particular set of cells in C. elegans—the M lineage, which gives rise to all the cells born post-embryonically that become part of the mesoderm, the middle germ layer of an animal. In the worm, these cells will ultimately become various types of muscle and non-muscle cells.
“In humans and other mammals, the mesoderm produces the heart, bones, blood, and muscles,” Liu explains. “So the question is, what’s so special about the precursor cell that has the potential to produce all these different types of mesoderm cells? What’s involved in regulating how it divides? Why do some of its progeny differentiate into one cell type, while some others differentiate into another cell type?”
Using molecular genetic techniques, the Liu lab put green florescent markers inside C. elegans M lineage cells so that the researchers could track each cell as the developing worms went through cell division. They found that the neighboring environment tells one cell to behave differently from its sister cell with a different neighboring environment; but they also discovered that cells inherit regulatory proteins from their mother cell.
“We identified a number of these regulatory proteins that tell the mother cell how to divide, how many times to divide, and when to stop dividing and become a muscle cell or a non-muscle cell,” Liu says. “Some of these proteins are present very transiently, only for a couple of hours. Once a protein has finished its job, it disappears quickly.”
“We identified a number of these regulatory proteins that tell the mother cell how to divide, how many times to divide, and when to stop dividing and become a muscle cell or a non-muscle cell.”
Liu and her colleagues are continuing to manipulate the proteins, mutating them in the worms and then looking at the consequences. “We are interested in understanding the underlying logic of how a complex lineage is built. We hope to achieve this by using molecular genetics to figure out if a protein is working with another protein or if it’s regulating the expression of other proteins, and so on,” Liu says. “A lot of the factors we identified that are important to the development of the M lineage have counterparts in humans that share similar regulatory relationships. So the lessons we learn in worms may be applicable to humans.”
A Mutant Worm, New Discoveries, and the Bone Morphogenic Protein (BMP)
While the researchers were studying cell differentiation in the worms, they accidentally cued in to a new area of research. They identified a mutant worm with a defective protein that affects the dorsal-ventral asymmetry of the M lineage. In a normal worm, when the M cell divides, one daughter cell ends up on the dorsal, or back, side of the worm and one ends up on the ventral, or belly, side. This process results in asymmetry as the dorsal cells ultimately produce fewer and different types of cells than the ventral. In the mutant worm, however, the cells that end up on the dorsal side behave just like the ventral cells. “It’s a really cool phenotype that’s leading us in a completely new research direction studying cell-cell signaling,” Liu says.
The Liu lab set out to discover what mechanisms regulate this dorsal-ventral asymmetry. Using a suppressor screen, an assay that uses a chemical to randomly create base pair mutations in the mutant worm’s genome, the researchers eventually zeroed in on a series of proteins in the bone morphogenic protein (BMP) signaling pathway.
In a fully functional signaling pathway like BMP, a signaling cell sends out a protein that binds to receptors on responding cells and transduces the signal to other proteins inside the cell. The intracellular proteins relay the signal to the nucleus and affect gene expression. The BMP pathway functions at many different times during development and in many different cell types.
“In humans, if you have defects in the BMP pathway, you can have a variety of defects, such as cardiovascular and bone formation problems or certain types of cancer,” Liu says. “The BMP pathway needs to be tightly regulated so signaling only happens at the right time and right place, and at the right level for the right duration.” The suppressor screen that the Liu lab carried out allowed them to identify a number of new factors that function to regulate BMP signaling. Importantly, all of these factors are conserved in humans, and mutations in some of these human proteins are associated with certain diseases or developmental disorders.
Two Proteins Identified: Their Role in the BMP Signaling Pathway
Among their many findings, Liu and her colleagues identified two proteins known as tetraspanins—TSP-12 and TSP-14—that are part of the BMP signaling pathway in C. elegans. They found that the proteins regulate the intracellular trafficking of the BMP receptors for cell-cell signaling. Normally the receptors are internalized to certain types of vesicles inside the cell and then recycled back to the surface to allow proper signaling. When the two tetraspanins are absent, the receptors are mis-sorted inside the cell and unable to recycle back to the cell surface, significantly dampening the BMP signaling pathway.
TSP-12 and TSP-14 have counterparts in humans, a family of five proteins whose functions are not well understood. “We’re really excited about this finding because it gives us clues about these proteins’ counterparts in humans in regulating BMP signaling and how they might be involved in regulating BMP signaling,” Liu says.
Liu’s research is driven by curiosity and the pull of new challenges. “You keep an open mind and sometimes things come your way, and you grasp the opportunity and try to learn as much as you can,” she says. “It’s challenging because a lot of the factors I studied early on are inside the nucleus. These signaling molecules I’m looking at now are either secreted outside the cell or are transmembrane or membrane associated proteins at the cell surface or in intracellular vesicles. I was more a molecular geneticist in the past. Now I have to learn biochemistry and high-resolution imaging, among others. But it’s intellectually exciting. I’m never bored.”