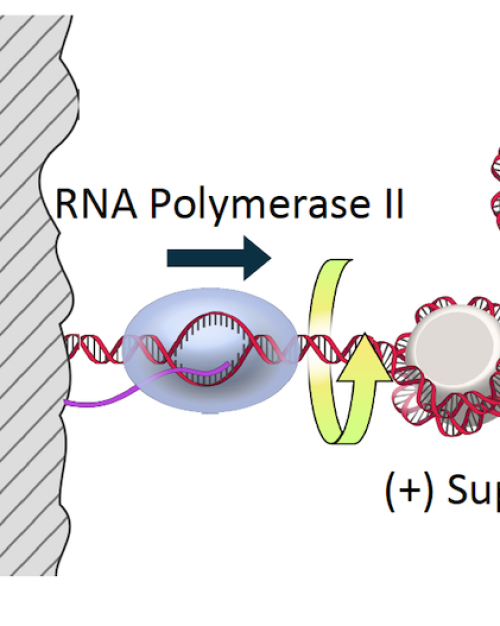
To develop more efficient next-generation materials for solar energy harvesting, researchers must learn to control the way molecules interact – their “coherence” when they absorb light. And to gain this control, they need methods of evaluation.
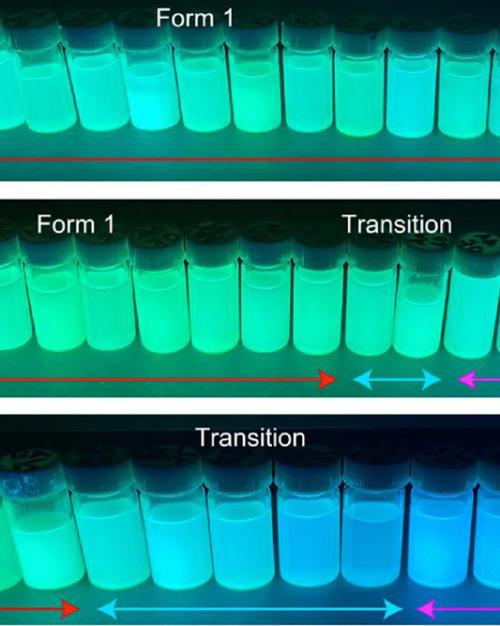
Andrew J. Musser and Phillip J. Milner, assistant professors of chemistry and chemical biology in the College of Arts and Sciences, have discovered that colors can help quantify the way energy moves through a specific type of crystal, called a metal-organic framework (MOF), in which light-sensitive molecules are arranged. The Milner group built different MOFs with two distinct structures based on the level of defects in each, an important discovery for the MOF field for its simplicity. Then, the Musser group evaluated the two MOFs under UV light; one glowed green, showing higher order and less energy emitted. One glowed blue, showing less order and more energy emitted.
The result gives a method for researchers to evaluate more complex materials for solar energy harvesting, which must allow energy to move in three dimensions.
“Enhancing Dynamic Spectral Diffusion in Metal-Organic Frameworks Through Defect Engineering” published in the Journal of the American Chemical Society Jan. 3. First authors are Arjun Halder, former postdoctoral researcher in the Milner group, and David C. Bain, doctoral candidate in the Musser group. As an undergraduate researcher, Stavrini Tsangari ’21 made significant contributions.
The paper is a foundational study toward Milner and Musser’s collaborative project “Controlling Quantum-Mechanical Coherence in Modular Framework Materials,” supported by a College of Arts and Sciences New Frontier Grant, to build next-generation materials for solar energy harvesting and quantum information science.
“The materials we’re working to develop are intended to increase the efficiency of silicon solar cells, or in fact any type of solar cell,” Musser said. “They will harness light in the blue side of the solar spectrum much more efficiently, resulting in a markedly higher power output. If we can get this to work, it would be transformative for solar energy generation.”
Organic semiconductors are governed by the way they’re packed together in a crystal, which is hard to control. The way the molecules end up in the crystal controls the behavior of the material.
Milner specializes in engineering MOFs by arranging organic molecules in a crystalline grid, like Tinker Toy components. By changing the size of the individual pieces and changing the angles at which they connect, he systematically tunes how the molecules interact. “Due to their excellent tunability, MOFs are promising for a range of applications relevant to a clean energy future, including solar energy harvesting,” Milner said.
With Halder in the lead, the Milner group discovered that through simple tweaking of the processing conditions when making MOFs, they could pack molecules into two different structural forms, essentially by controlling the defects in the material.
Halder discovered that the two different MOFs glowed different colors under ultraviolet light. He asked Bain, in Musser’s group, to help him understand what that meant.
“Typically, the color that a molecule or material glows is a read-out of its electronic properties,” Musser said. “A different color tells you that something fundamentally new has happened in this material; the electrons are absorbing and emitting light in a different way.”
The Musser group applied spectroscopic tools they’ve developed to analyze the two MOFs. Where there are more defects, there’s more disorder, Musser said, and higher disorder in a material changes its ability to manage energy.
“Mapping that energetic disorder with our spectroscopic measurements showed that when you have more disorder, you can see this in real time as the energy migrates through different sites and changes the color of light that it emits,” Musser said. “When there’s more disorder, you have more energy migration, and that changes the color of the framework.”
The MOF that glowed green is more ordered, with most of the molecules behaving in the same way and lower energy emitted, Musser said. The blue photons of the other MOF indicate higher energy states, more disorder, and more energy being emitted.
In the context of solar energy, these particular MOFs are not themselves useful, but the researchers are already using what they’ve learned about tuning molecule interactions and measuring energy migration to analyze more complex materials.
“In any application of molecular materials for harvesting solar energy, one of the key steps is that the energy has to move around in three dimensions,” Musser said. “In a solar cell, wherever the light gets absorbed, that generates a state of excited electrons. They have to move to some kind of interface where the electrons get separated and turned into energy. Sometimes that distance is very short, like 10 nanometers. But it can be 10 times longer or even more.”
Read the story in the Cornell Chronicle.