Yeast – that simple organism essential to making beer and bread – has revealed for Cornell researchers a key mechanism in how genes are controlled.
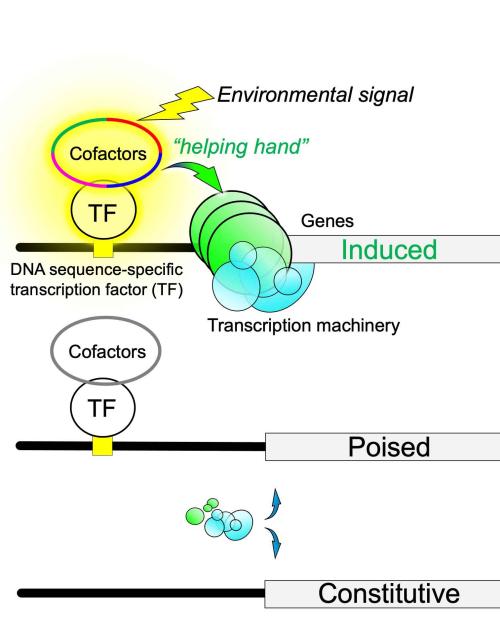
Gene transcription – the elaborate process that our cells use to read genetic information stored in DNA – was long thought to be turned on only when certain regulatory factors traveled to specific DNA sequences. In new research, published Oct. 27 in Genes & Development, a team of Cornell researchers discovered that certain genes have their transcription regulatory factors and cofactors already in place, but in a latent state. With the appropriate signals, these “poised” genes become highly active.
Using CRISPR techniques, the researchers removed parts of the yeast transcription machinery to systematically examine the role they play in regulating genes. Yeast and humans have mostly the same molecular machinery to regulate their genes, so yeast provides an excellent model for understanding gene regulation in humans.
“It’s like the game of Jenga, where you remove a wood block from a tower of blocks and see if the whole thing crashes down. That’s how we learn how protein machines work inside cells,” said B. Franklin Pugh ‘83, the Greater Philadelphia Professor of Molecular Biology and Genetics in the College of Arts and Sciences.
The researchers identified two classes of yeast genes, based on how they are regulated. The first and largest group provides basic housekeeping functions, allowing the cells to live and grow. These genes are always “on” at very low levels because the transcription machinery has a hard time finding its way to each gene.
The second class, the “inducible” genes, has a whole entourage of proteins assembled nearby. When triggered by environmental signals this poised entourage provides a guiding hand to transcription machinery. This results in high levels of induced transcription.
“The value of being poised is that certain genes, like environmental response genes, can rapidly respond to a changing environment; for example, when yeast encounters and metabolizes bread sugars, causing the bread dough to rise,” Pugh said. Similar metabolic processes happen in human cells when food is eaten.
Pugh has been researching gene regulation for more than 30 years. As a student at Cornell, the idea of how our genes are regulated – so central to biology and so unknown – fascinated him, and he’s spent his life trying to understand it.
“With this paper, we finally got to the core question of how do these gene-specific transcription factors that are sensing the environment recruit the core transcription machinery,” he said. “We could not completely answer the question in this paper, but we got solid insight into how that process works.”
In previous related work, Pugh mapped precise binding sites of more than 400 different chromosomal proteins in the yeast genome, most of which regulate the expression of genes.
“That paper shed significant light on understanding how all these proteins come together and work together to read and regulate genes,” Pugh said. The new research builds on that work, delving deeper into understanding the architectures of proteins and the machinery at genes.
“Building upon years of existing research and combining them with modern and elegant genomics tools helped us in filling gaps in the current knowledge as well as in making new discoveries,” said first author Chitvan Mittal, research associate at the Baker Institute for Animal Health in the College of Veterinary Medicine.
In addition to Pugh and Mittal, co-authors included William Lai, assistant research professor (A&S) and Olivia Lang, doctoral student in the field of computational biology, who managed the analysis of the thousands of data sets generated by the research.
The work was supported by a grant from the National Institutes of Health.
This story also appeared in The Cornell Chronicle.