Photocathodes are materials that emit electrons when illuminated by light, and are vital to the performance of some of the world’s most powerful particle accelerators.
But due to poor crystalline properties, photocathodes have yet to realize their full potential. Cornell researchers are addressing this limitation.
A team of researchers at Cornell’s Center for Bright Beams, a National Science Foundation Science and Technology Center, has developed a technique to create a single-crystal alkali antimonides photocathode, with an efficiency up to 10 times higher than its predecessors.
Their work is described in a paper published March 18 in Physical Review Letters. The corresponding author is Jared Maxson, Ph.D. ’15, the Joyce A. Yelencsics Rosevear ’65 and Frederick M. Rosevear ’64 Assistant Professor of Physics, in the College of Arts and Sciences.
Other co-authors include Tomas Arias, professor of physics (A&S); Melissa Hines, professor of chemistry and chemical biology (A&S); Darrell Schlom, the Herbert Fisk Johnson Professor of Industrial Chemistry in the Department of Materials Science and Engineering (ENG); and Kyle Shen, the James A. Weeks Professor of Physical Sciences in the Department of Physics (A&S).
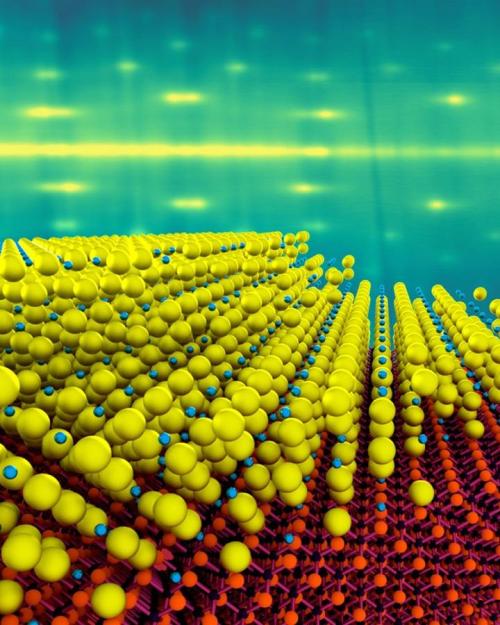
Some of the most efficient photocathodes are made from alkali antimonide materials. While used in a variety of accelerators, these materials have not yet reached their theoretical limit due to poor crystalline properties. This can result in surface roughness, leading to a degradation of the emitted beam quality and, ultimately, a degradation in accelerator performance.
Additionally, they are made of extremely reactive materials, which makes them difficult to grow and characterize precisely.
While the atomic ingredients in the new photocathodes are the same as in existing types, Maxson’s group was able to grow the materials as a single crystal, with a thickness of just 4 nanometers – approximately 10 times thinner than existing materials, which means they should respond about 10 times faster.
This would enable unprecedented short electron pulses for time-resolved science at the scale of 10 femtoseconds – faster than typical atomic motion.
“These electron source materials have been around since the days of Einstein,” Maxson said. “His Nobel Prize-winning work actually describes the photoelectric effect these sources utilize. They are extremely efficient and make a lot of electrons, but at the atomic level, they are completely disordered, which we know limits their performance.”
At the atomic scale, any defects can not only limit the number of electrons emitted, but can make the electrons come out at unreliable angles. In order to grow the thin materials while maintaining order of the electrons within them, the team made use of the molecular-beam epitaxy system at the Platform for the Accelerated Realization, Analysis and Discovery of Interface Materials (PARADIM) to deposit a single-crystal layer of cesium antimonide, just 4 nanometers thick, onto a substrate of silicon carbide.
Using this technique, the team “grew” the alkali metal one atomic layer at a time, allowing them to lock the orientation of the atoms to create a single-crystal film.
While the beam originates at the photocathode, the brightness and overall effectiveness of the electron beam must be maintained right up until it interacts with its target. By overcoming the limitations throughout the beam production and transport process, researchers are able to produce even brighter electron beams that are more tightly focused, enhancing particle accelerator performance.
Maxson’s lab is not just making the sources of electrons. They also are driving one particular application of the sources: ultrafast electron diffraction. This technique uses extremely short pulses of these dense, bright beams to make movies of atomic motion. There’s one catch: Atomic motion happens at the scale of a trillionth of a second, so it’s important these beams be both short in time and small in size.
“A lot of diffraction apparatuses can make beams that are short in time,” said Maxson, co-author on another recent publication describing the ultrafast electron diffraction system. “But we’re also making them extremely small in spatial size. The resulting beams are not just short, they are really dense, and the only way we can achieve that is with a very good photocathode and pristine transport of the beam from the source to the sample we’re studying.”
Maxson said a good photocathode, paired with the ultrafast electron diffraction apparatus at Cornell, can deliver an unmet need in the scientific community.
“A lot of the new materials that are being explored in materials science communities around the world can only be made at the single micron scale,” he said. “We cram our beam into a small spatial scale in order to unlock the ability to view these materials with time-resolved electron diffraction. Cornell is driving this research in a way that has not been done before.”
Rick Ryan is a science communicator for Cornell’s Laboratory for Accelerator-based ScienceS and Education (CLASSE).
Read the story in the Cornell Chronicle.