A cellular biology “mystery” is closer to being solved, thanks to sleuthing in the lab of Jeremy Baskin, assistant professor and Nancy and Peter Meinig Family Investigator in the Life Sciences in the Department of Chemistry and Chemical Biology and the Weill Institute for Cell and Molecular Biology.
Last year, Baskin and doctoral student Timothy Bumpus reported on a new method for imaging the versatile lipid phosphatidic acid (PA), a product of the enzyme phospholipase-D (PLD) that catalyzes a variety of cell-signaling events and figures in several diseases, including cancer.
But their two-step imaging technique had a drawback: For the second step – fluorescent tagging of the molecule – to occur, they had to essentially kill the cells, so the ability to track the molecule’s movement and reactions downstream was lost.
Baskin and Bumpus now report a major improvement to their fluorescence imaging technique: the use of different chemistry that preserves the cells during labeling, so they can be studied as they continue to grow.
Their work, “Clickable Substrate Mimics Enable Imaging of Phospholipase D Activity,” was published Oct. 4 in ACS Central Science.
The pair call their new technique IMPACT – Imaging Phospholipase D Activity with Clickable Alcohols via Transphosphatidylation – and hope it has just that on the biochemistry world.
“We’re excited by some of the interesting biological observations that we’ve been able to see with the method,” said Baskin, who has filed paperwork for a provisional patent on the technique. “In addition to not killing the cells at the end of the labeling process, our labeling now has better sensitivity, which means our detection limit is expanded.”
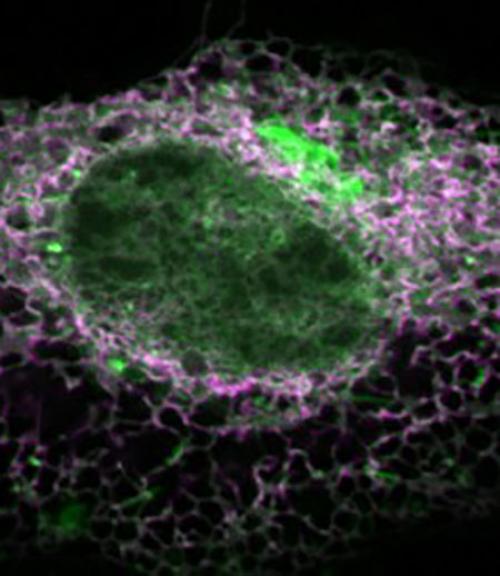
The diversity of physiological and pathological signaling outcomes produced by PA is still a mystery, due mainly to the inability to visualize the dynamics of its production within cells. The Baskin Lab’s IMPACT method addresses that deficiency.
Imaging began with alcohol stimulation of PLD activity in live HeLa cells to generate PA, which is then fluorescently tagged. HeLa cells are a cell line used in research and derived from cervical cancer cells taken in February 1951 from Henrietta Lacks, who died of the disease in October of that year.
The imaging work reported on last year used copper, which is toxic and stops cells’ biological reactions, in the tagging process. This time around, Baskin used a copper-free chemical reaction to preserve the cells and track further movement.
Additionally, chemical stimulation of the cells wasn’t repeated during the tagging phase this time around, as it was in the group’s previous study. That revealed more natural, or “resting,” enzymatic activity: pools of PLD activity in “expected and unexpected locations,” Baskin said, and variability from cell to cell.
“When we stimulate the cells strongly, all of the cells get activated roughly equivalently,” Baskin said. “But when we look at the resting population of cells, we identify a few – around 1 percent – that have really high activity.
“The fact that we can do this labeling,” he said, “and see the variability from cell to cell in the population enables us to analyze what’s different about them.”
“Now,” Bumpus added, “what we want to be able to figure out is, what exactly is that difference?”
Future work will also investigate PLD’s role in cancer cell growth; PLD has long been linked to immune cell function and to biological processes required for tumor growth and cancer metastasis. Baskin hopes his work can contribute to developing better PLD inhibitors used in cancer therapy.
“It will allow us to understand in what context the inhibitors will be and won’t be effective,” he said. “Any time you can use basic research to inform on mechanisms, it could save lots of money in clinical trials.”
Baskin says both the findings and the method by which they were achieved are notable.
“From a fundamental point of view,” he said, “what we see when we develop these new chemical tools is that we’re revealing different types of information about this reasonably well-studied system, compared to what’s already known.”
This work was supported by the National Institutes of Health, as well as a Beckman Young Investigator Award to Baskin and a National Science Foundation Graduate Research Fellowship to Bumpus.