Cornell researchers have discovered there is a division of labor among immune cells that fight invading pathogens in the body.
The study, published June 14 in the journal Cell, finds for the first time that fetal immune cells are present in adults and have specialized roles during infection. In fact, the first immune cells made in early life are fast-acting first responders to microbes in adulthood.
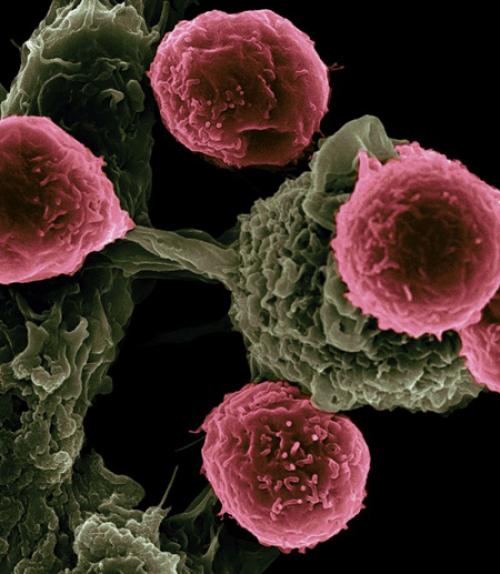
T cells (pink) scanning host cells (green) for evidence of infection.
These immune cells – called CD8+ T cells – come in fetal and adult varieties, which originate in separate parts of the body and are hardwired with intrinsically different properties. The current paradigm is that, around the time of birth, the body switches from making and using fetal T cells to adult T cells to defend itself. But Cornell researchers used a unique study design to show that fetal T cells persist into adulthood and have different roles than adult cells in fighting infection.
“This discovery has led to the new idea that we might be able to predict how individuals will respond to infection based on how many fetal cells are present in the adult pool and isolate the fast-acting fetal-derived cells for certain therapeutic interventions, such as infections and cancer immunotherapy,” said Brian Rudd, associate professor of immunology in the College of Veterinary Medicine and the paper’s senior author. Norah Smith ’02, Ph.D. ’11, a research associate in Rudd’s lab, is the paper’s first author.
T cells are made in an organ called the thymus, which sits above the heart. In order to make a T cell, the body requires a stem cell, but the origin of these stem cells changes through development. The first stem cells that colonize the fetal thymus come from the fetal liver and give rise to fetal T cells. Around the time of birth, a new wave of stem cells are made in the bone marrow, which then colonize the thymus and give rise to adult T cells.
“What we found is that fetal and adult T cells are intrinsically different,” Rudd said. “The reason for that is they derive from different stem cells, which are genetically distinct.”
In adults, newly formed T cells recognize a signature protein on a pathogen when they first encounter it. That signal then activates the T cells and equips them to fight and proliferate up to 15 times, producing up to 10 million cells in a week. Once the pathogen has been cleared, most of those adult T cells die, but up to 10 percent survive and are stored in a pool of memory cells, allowing for a rapid recall response if that same pathogen were to strike again.
Fetal-derived cells, on the other hand, are generalists and do not form into memory cells. They respond to inflammatory signals and activate faster than adult T cells, allowing them to provide a broad swath of protection against pathogens they don’t specifically recognize, Rudd said.
“It’s the way that the immune system hedges its bet: It has cells that can respond at different rates,” Rudd said.
Previously, scientists had no way of tracking these different types of cells. In the study, the Rudd lab used a special “time-stamp” mouse that allowed them to map the fate of these cells throughout the mouse’s life. A drug (tamoxifen) injected at various stages of life marks the waves of T cells made during different stages of development. The system allowed the researchers to see which cells were present during adulthood.
“We were able to map cells produced at different stages of life and track their numbers and functions over the life span,” Rudd said. “This led to the discovery that there are developmental layers to an immune response, which has previously gone unnoticed.”
Future work will explore how genetic and environmental factors, such as diet and gut bacteria, may alter the developmental layers in the immune system.
Co-authors include researchers from Andrew Grimson’s lab in the Department of Molecular Biology and Genetics and mathematicians from Miles Davenport’s lab at the University of New South Wales in Sydney, Australia.
The study was funded by the National Institutes of Health and the National Health and Medical Research Council in Australia.
This story also appeared in the Cornell Chronicle.